
Acid Digestion Of Minerals
Every time we eat, our bodies break down our food through digestion. In mammals, this process is accomplished using both mechanical breakdown (ex., chewing) and chemical decomposition (ex., stomach acid). In the lab, scientists use a similar concept to render solid samples into solution using a process called acid digestion. Acid digestion has many applications, including analyzing the composition of rocks, ores, minerals, and soils, which aids in understanding the geological and geochemical processes that led to their formation and predicting the location of commodity and high-value deposits. Acid digestion takes these solid rock/soil/mineral samples and processes them into liquid form for analysis through techniques of inductively coupled plasma-mass spectrometry (ICP-MS) or atomic absorption (AA).
Solid To Liquid: The Process Of Acid Digestion
The first step in many acid digestion workflows is grinding the solid rock, ore, mineral, or soil samples to 200-230 mesh. This creates fine powders that are more homogeneous and allows for reduced sample sizes in the remaining processing steps, as compared to the large solid samples. Smaller particles with greater surface areas are also more susceptible to dissolution by commonly used acids during subsequent steps.
Following grinding, the powdered sample is subjected to digestion using acids such as hydrofluoric (HF), perchloric (HClO4), hydrochloric (HCl), sulfuric (H2SO4), nitric (HNO3), or a combination such as aqua regia, prepared as a 3:1 ratio of HCl to HNO3. The acid(s) required for an application depends upon the sample type, as elements or minerals may be more or less soluble in certain acids. Regardless of the acid(s) selected, acid purity is paramount to ensure that elemental contaminants are not introduced into the samples, which may result in false positives. Similarly, the types of vessels and crucibles used must be compatible with the acid digestion process to avoid the potential leaching of contaminants into the samples.
Once the appropriate acid is chosen for the application, the actual digestion of the powdered sample can be accomplished using various techniques, including open and closed vessel digestion and microwave processing. Open vessel digestion occurs by heating the ground sample in the selected acids within an open container, while closed vessel methods heat samples in sealed containers. Microwave digestion may be either open or closed, and, as the name implies, microwaves are used to heat the sample. In general, the increase in pressure during the closed vessel method and rapid microwave-induced heating reduces digestion times compared to the open vessel method.
Acid Digestion Can Create Toxic Particulates And Vapors
Most steps of the acid digestion process can create a potentially dangerous environment. Sample grinding releases small particles and dust into the air that can be inhaled by operators or others in the area. While a little rock dust may seem harmless, most rocks and soils on Earth contain silica or silicon dioxide. Inhalation of silica dust over time can cause an irreversible lung disease called silicosis, which has recently been brought back into the spotlight following the increased popularity of engineered stone (ex., quartz countertops).1,2 In addition to particulates, operators completing acid digestions must also be cognizant of vapor production. All digestion techniques can generate hazardous, toxic, corrosive, and irritating acidic vapors and byproducts. For example, many digestions use hydrofluoric acid to decompose silica and silicates.3
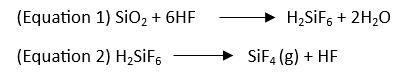
Silicon dioxide reacts with hydrofluoric acid to form silicic acid (H2SiF6) (equation 1), which dissociates into gaseous silicon tetrafluoride (SiF4) and hydrogen fluoride (HF) (equation 2). Hydrofluoric acid, hydrogen fluoride, and silicon tetrafluoride are all toxic and illustrate the safety and health hazards associated with digestion.
Operator Protection During Acid Digestion
Proper ventilation is clearly an essential consideration during the digestion process to protect operators and the work environment. Enclosures such as the AirClean® Systems PowderSafe™ series are designed to provide enclosed areas for grinding smaller samples and prevent inhalation of dust and particulates by operators. PowderSafe enclosures pull air from near the operator across the workspace to the back or top of the hood. The air is then filtered through two high-efficiency particulate air filters (HEPA) to remove dust and particulates prior to exhaust into the work area.
To protect operators from hazardous vapors, operators should complete acid digestion procedures within total exhaust fume hoods fitted with wet scrubbers. In addition to protecting the operator, the wet scrubber is an important consideration for helping to protect the environment and surrounding structures adjacent to the fume hood exhaust stack. The wet scrubber minimizes the corrosive pollutants released from the building exhaust systems into the atmosphere. The AirClean Systems AirMax Fume Hood With Wet Fume Scrubber protects the operator from inhaling acidic vapor and gas during the open vessel digestion process and after unsealing closed vessels or venting microwave digestion. Airflow draws the toxic, corrosive gases and vapors into the AirMax baffles and up into contact with water-washed high surface area media, where it dissolves and drains into a holding tank. An optional caustic dosing system neutralizes the acidic tank contents and the fluid is recirculated and continues to capture acidic vapors based on their water solubility. Perchloric acid digestions require the optionally-fitted spray bar to ensure residual perchloric acid and volatile metal perchlorate salts are washed into the holding tank
Processing rocks, minerals, ores, soils, and sediments for acid digestion presents chemists with both safety and scientific challenges. The AirClean Systems PowderSafe enclosures and AirMax Fume Hoods with Wet Fume Scrubber provide safety solutions for acid digestion laboratories. In a safe laboratory environment, chemists can focus their energy on the scientific challenges of developing efficient and effective acid digestion workflows.
References
- J. Fazio, et. Al. “Silicosis Among Immigrant Engineered Stone (Quartz) Countertop Fabrication Workers in California.” JAMA Intern. Med. (2023) 183(9):991-998.
- Occupational Safety and Health Administration. “Memorandum: Respirable Crystalline Silica Focused Inspection Initiative in the Engineered Stone Fabrication and Installation Industries.” (2023). Accessed from: https://www.osha.gov/laws-regs/standardinterpretations/2023-09-22
- V. Balaram; K.S.V. Subramanyam, “Sample Preparation for Geochemical Analysis: Strategies and significance”, Advances in Sample Preparation (2022). 1(100010 (Science Direct/ Elsevier).


