
Reflux
It’s Friday night. After a long week, you’re looking forward to a relaxing movie night and a plate of your favorite pasta. You put a pot of water on the stove to boil and decide to start watching a movie while you wait. You tell yourself you will just pause it once the water boils. Suddenly, the movie is almost over and you remember the pot of water on the stove. Rushing into the kitchen you find that the water is boiling, but half of the water is gone; lost to evaporation. There’s not enough boiling water remaining to cook your pasta. Feeling annoyed, you begrudgingly add more water to the pot and start again. If only you had your own refluxing apparatus, all of this could have been avoided.
Reflux: A Solution to Chemical Evaporation
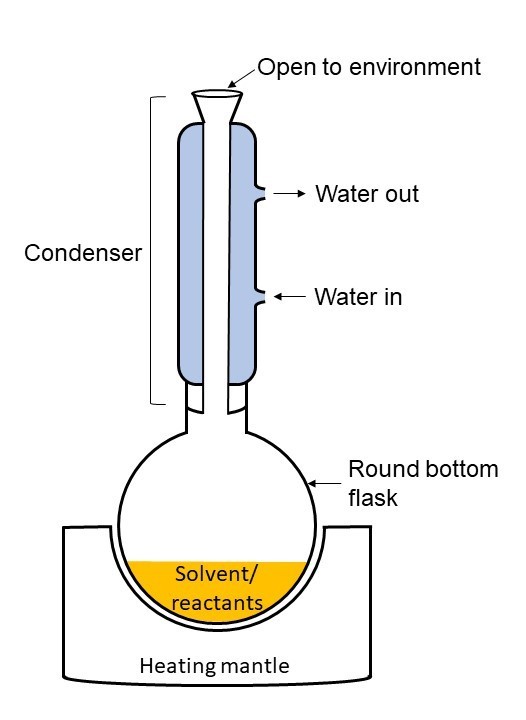
Figure 1. Refluxing Apparatus
While evaporating pasta water is an inconveience most people have dealt with at least once, mitigating evaporation during chemical reactions is a daily occurrence for some chemists. Many chemical solutions must be heated to a high, stable temperature, sometimes for hours at a time, to provide enough energy for the reaction to occur in a timely manner. Unfortunately, similar to forgotten pasta water, heating reactions to a high temperature for a long time can cause a large volume of solvent/reactants to evaporate. Evaporation reduces the amount of chemicals available to react in the flask and the amount of reaction product. To combat this issue, scientists developed a process called reflux. During reflux, chemical vapors from boiling solvent/reactants are captured on a cooled surface and drip back into the solvent vessel allowing boiling to continue for up to several hours without reducing the amount of solvent/reactants in the vessel. Typically, to begin the process, solvent is added to a round bottom flask equipped with a magnetic stirring bar. The round bottom flask may be set within an electric heating mantle or immersed partially in an oil bath. The chemical reactants are added to the flask and then a glass reflux condenser is attached vertically to the round bottom flask (Figure 1). Condensers come in may shapes and sizes, but all contain a hollow center. This hollow center can be shaped like a straight tube in the center or a coil-like structure. Water or glycol is then routed upwards through the condenser and out via tubing to provide a constant flow of cool water/glycol through the condenser for the duration of the reaction. Once setup is complete, the solvent/reactants in the round bottom flask are heated to the solvent boiling point. Solvent vapors then rise into the hollow center of the reflux condenser. When the vapors come in contact with the condenser, they begin to cool and condense on the inside wall of the condenser, similar to how water vapors in the air condense on the outside of cold glass of lemonade on a hot, humid summer day. The condensed solvent then drips back into the round bottom flask with the remaining solvent and reactants.
Reflux Applications

Figure 2. Soxhlet Extraction Apparatus
Reflux is ubiquitous to academic, pharmaceutical and industrial laboratories engaged in organic synthesis of both small molecules and polymers. Although typically found in organic synthesis, reflux also plays an important role in Soxhlet extraction, a process used for purifying organic chemicals by dissolving impurities into the refluxing solvent. Likewise, it can also be used to extract a desired material from an impure source, provided the desired material is soluble in the solvent and the impurities are not. The Soxhlet extraction apparatus is similar to the refluxing apparatus described above but has a Soxhlet extractor situated between the round bottom flask and reflux condenser (Figure 2). The Soxhlet extractor allows solvent vapor to pass upwards into the cooling condenser. However, instead of falling back into the round bottom flask, the liquid droplets fall into a permeable thimble containing the material requiring purification. Once the volume in the thimble reaches a threshold, the solvent drains back into the round bottom where it begins to boil again to continue the extraction.
Ductless Fume Hoods Prevent Chemical Vapor Exposure During Reflux
During reflux, chemical vapors and fumes created from boiling solvent and/or reactants are captured by the condenser. In an ideal system, chemical vapor capture would be 100% efficient, so that no vapors escape from the reflux condenser into the environment. However, rarely does a system reach 100% efficiency. Over time, even small amounts of vapor released by a highly efficient system can start to add up, especially in smaller spaces. Further, mishaps during the reaction, overheating, or spills while adding chemical reactants to the solvent flask mid-reaction could release chemical vapors outside the reflux apparatus. Solvents used in reflux and Soxhlet extraction vary considerably, but are often organic and can be hazardous to the operator’s health. To minimize the potential for exposure, reflux with hazardous reactants, solvents, or products should be completed in a fume hood. A ductless fume hood designed to pull air from near the operator across the workspace in a horizontal laminar fashion can help protect the operator and environment from hazardous fumes or nuisance odors. In these ductless fume hoods, the contaminated air is then pulled to the back and top of the hood and passes through an activated carbon filter to remove chemical fumes before being exhausted back into the room. Reflux has become a tried and true solution to chemical evaporation in academic and industrial laboratories. It’s use across many applications suggests that it will not be removed from the laboratory toolkit anytime soon. However, scientists should take precautions when completing reflux experiments that could release hazardous chemical vapors. Ductless fume hoods can provide an additional margin of safety to prevent operator and environmental exposure, at least until more automated and/or enclosed reflux systems are developed. Who knows maybe the development of automated systems will spur an at home reflux apparatus that the forgetful pasta makers among us desperately need.




